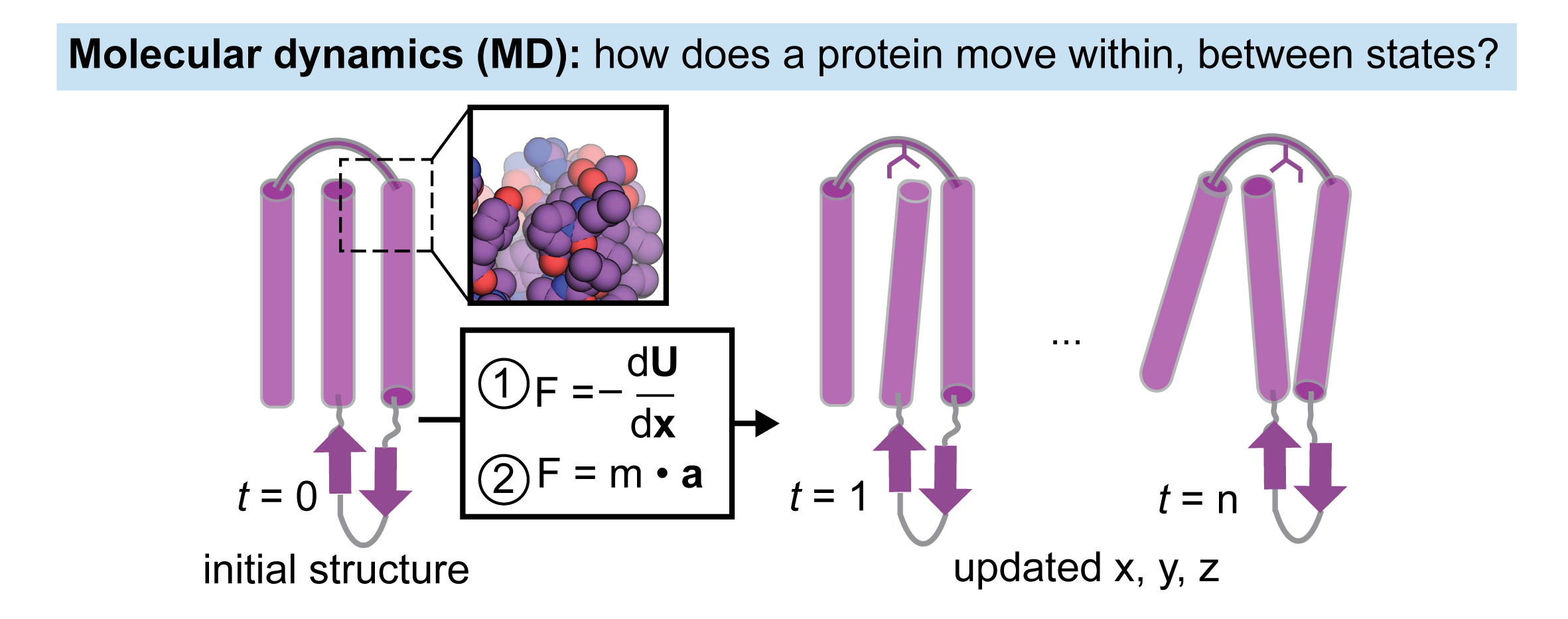Motivation
Nearly half of all FDA-approved therapeutics target membrane proteins, yet these drugs bind only a small fraction of possible targets, signaling a community-wide need to investigate understudied and undrugged portions of the membrane proteome.
In our lab, we primarily study G protein–coupled receptors and solute carrier transporters. We seek to identify:
the conformational states proteins sample, and how these states enable function
the atomic-level motions that underly transitions between these states
how ligands, sequence variation, and protein oligomerization state influence conformational equilibria
Ultimately, we aim to use this mechanistic information to develop new pharmacological strategies for modulating and engineering protein function.
Systems
Allosteric regulation in multidomain membrane proteins
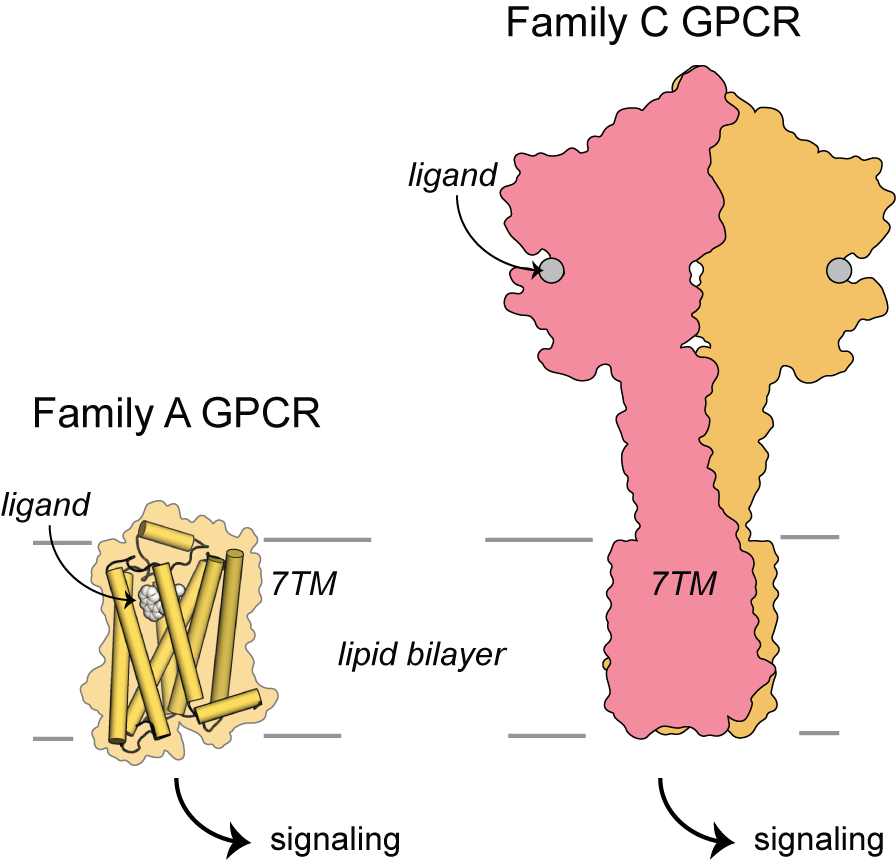
The 800 G protein–coupled receptors (GPCRs) make up the largest family of membrane proteins and share a common seven-transmembrane helix (7TM) fold. Their sequence-diverse ligand binding pockets recognize a wide array of ligands––from photons and small molecules to peptides and proteins––and induce changes in receptor shape (conformation) to induce intracellular signaling cascades.
Some subfamilies of GPCRs, including the Family B and Family C GPCRs, also possess large extracellular domains. Ligands that bind within these extracellular domains, including many neurotransmitters, induce conformational changes that must be ‘sensed’ tens of nanometers away, on the intracellular side of the membrane. How do distant conformational changes within these extracellular domains affect transmembrane domain conformation and function? Do extracellular domains limit or alter the types of conformational rearrangements accessible to GPCR 7TM domains?
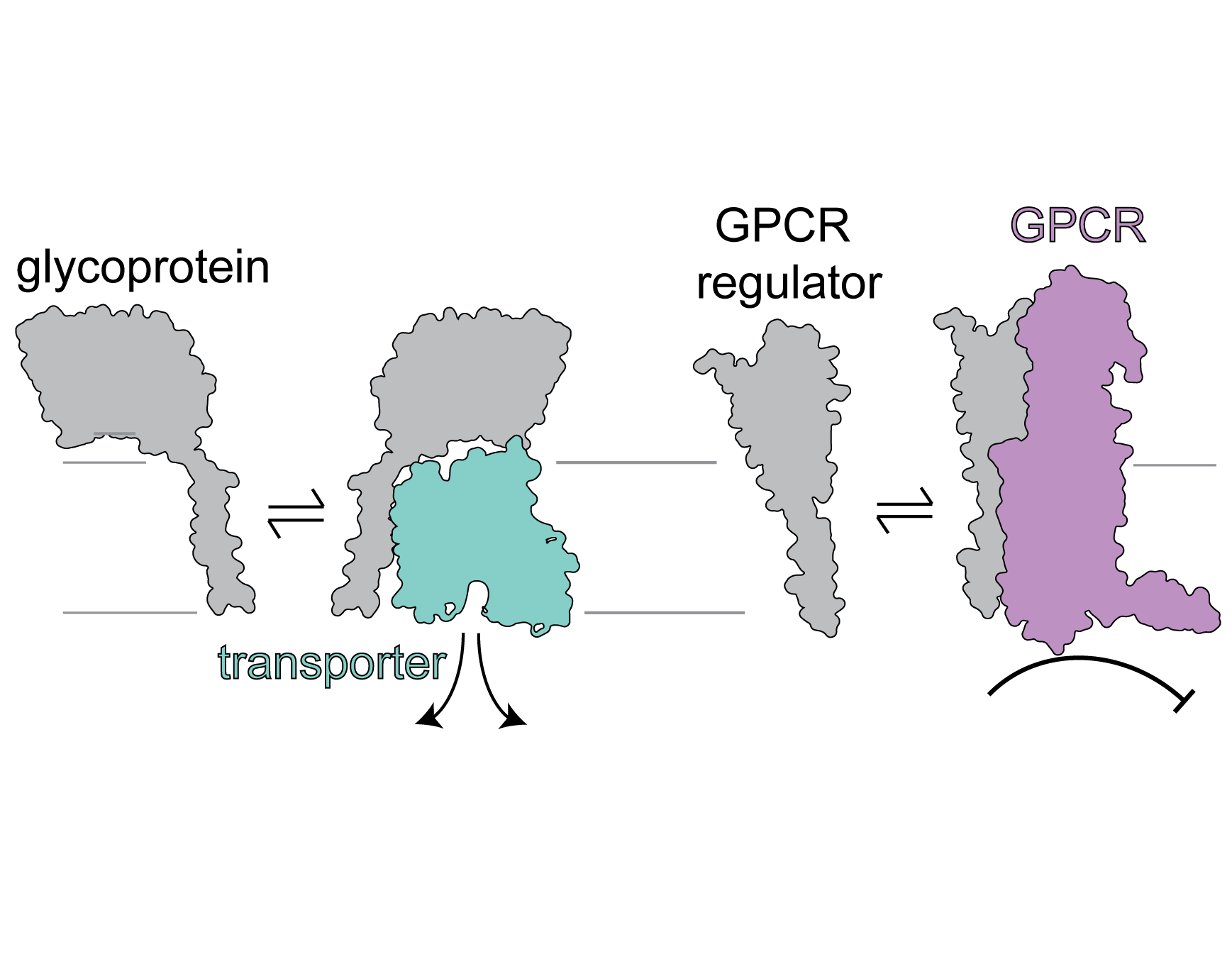
Endogenous regulators of membrane protein function
A wide variety of membrane proteins, including ion channels, receptors and transporters, undergo functional regulation by ‘auxiliary’ membrane protein binding partners, including a variety of single-pass (1TM) membrane proteins. These interactions may account for the failure of certain drug candidates in clinical trials, and the discovery and characterization of such interactions may open new avenues for therapeutic modulation. What sequence features give rise to specificity between single-pass TM modulators and their receptor targets in the membrane? Can we exploit these properties to design new modulators of transmembrane protein function?
Approaches
We iteratively integrate information derived from experimental and computational biophysical approaches to develop a detailed understanding of how proteins work at the atomic level:
Initiate molecular dynamics (MD) simulations from different starting structures (conformations) to identify trajectories that transition between states
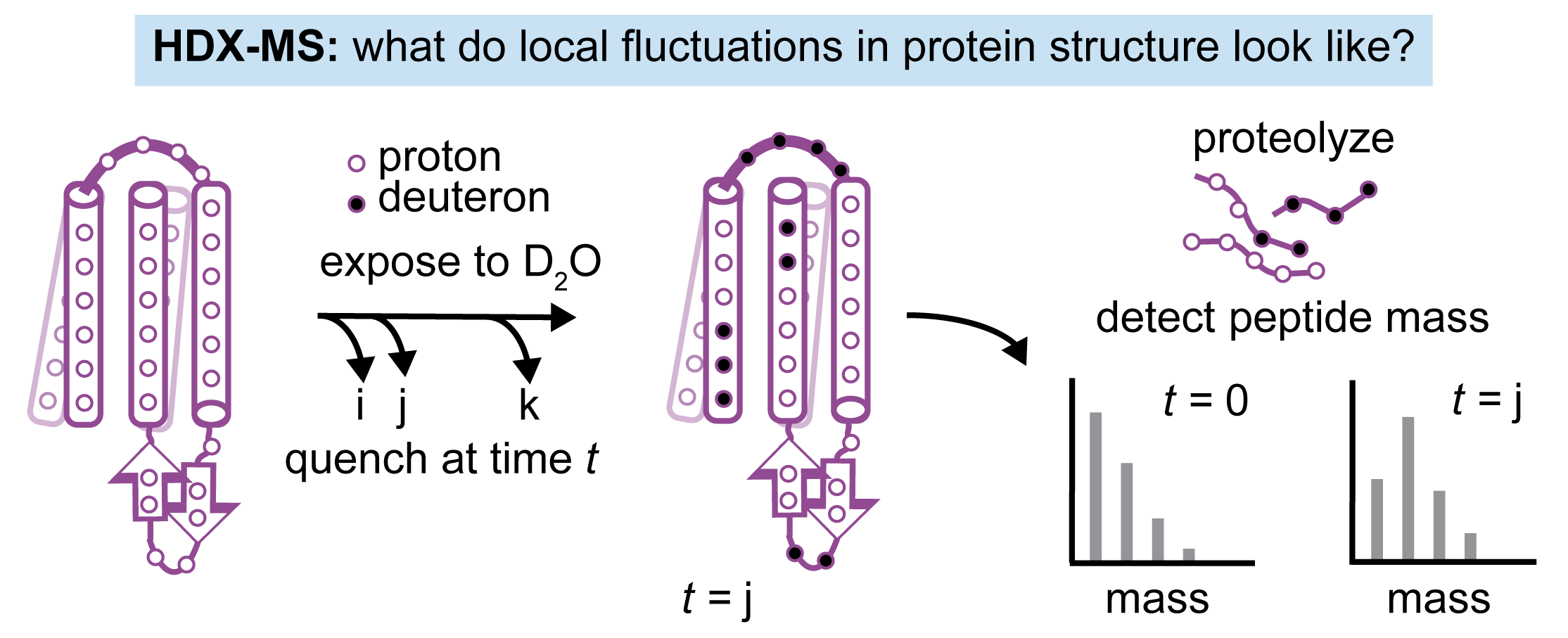
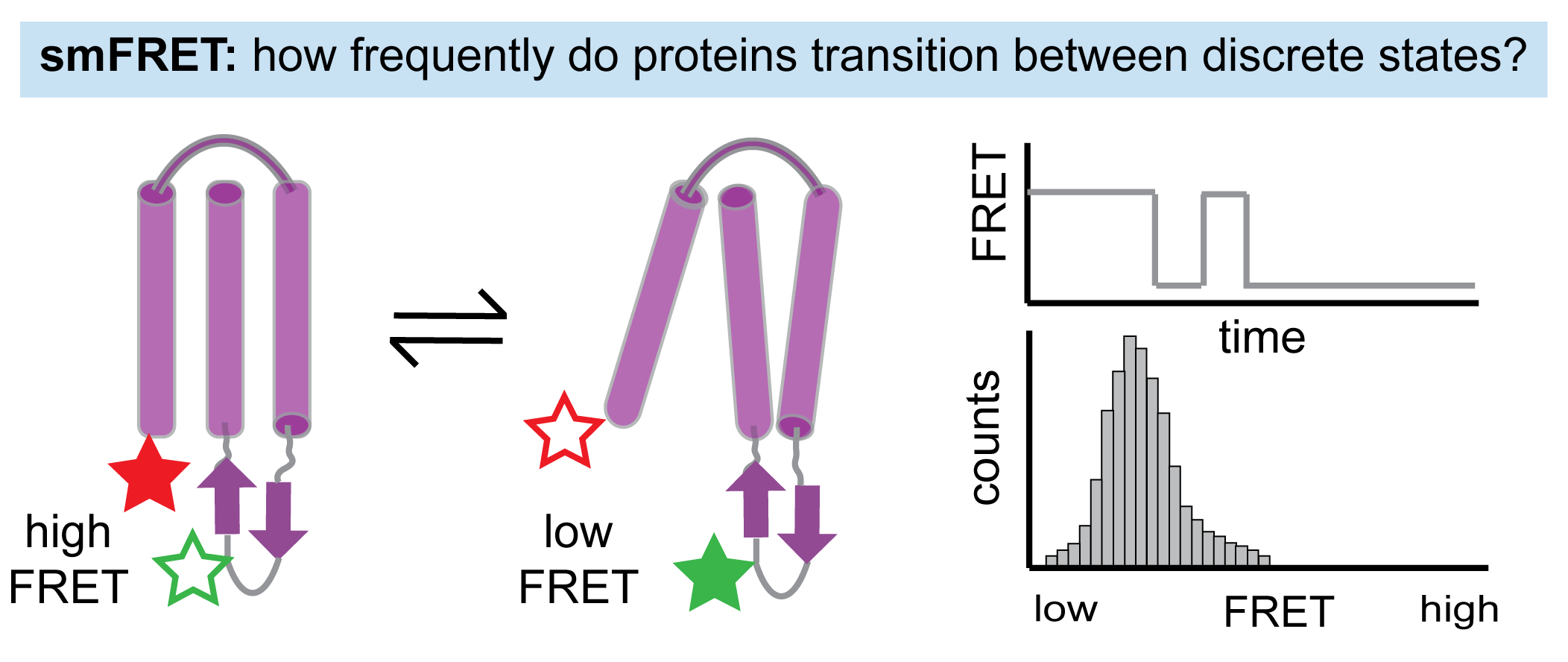
Probe simulation-based mechanisms through systematic introduction of perturbations (mutants, ligands) in hydrogen-deuterium exchange and single-molecule fluorescence experiments that capture molecular motions on different timescales and with different spatial resolution